Tips for Best Practices on Management of Abnormal Vaginal Cytology and HPV Tests
Post date: November 15, 2024
Reviewed by: Helen Cejtin, MD, MPH
Vaginal cytology and/or high-risk human papillomavirus (hrHPV) testing after total hysterectomy for benign disease is not recommended, except when the patient has a history of high-grade cervical dysplasia within the past 25 years. However, these tests are often performed without a valid indication. Practitioners are then challenged with how best to manage abnormal results.
Vaginal cancer is a rare, HPV-related gynecologic malignancy with a reported incidence rate of 0.4–0.6 per 100,000 women. Cytologic abnormalities and HPV infection are both relatively common in individuals who have undergone hysterectomy. HPV infection of the vagina is found with similar frequency as HPV infection of the cervix and the prevalence of hrHPV is similar between individuals with and without hysterectomy.
Since vaginal cancer is so rare and the accuracy of vaginal hrHPV testing and cytology to predict histologic HSIL/VaIN2/3 is not very well defined in the literature, a conservative approach to management of abnormal vaginal tests is recommended.
Healthy asymptomatic individuals with negative hrHPV testing and a negative cytology are at extremely low risk and do not require future testing if they have had hysterectomy for benign disease and have no history of cervical precancer or cancer.
Individuals with a history of cervical precancer should be followed with surveillance tests for at least 25 years after their treatment as per ASCCP management guidelines. Individuals with a history of cervical cancer should be followed with surveillance tests as per NCCN guidelines.
For individuals with ASCUS, cytologic LSIL, or NILM hrHPV+ results, vaginal cotest or repeat cytology in one year is recommended (see figure1)
In individuals with ASC-H, HSIL or AGC cytology results, vaginal colposcopy with biopsy of any lesions is recommended (see figure 1)
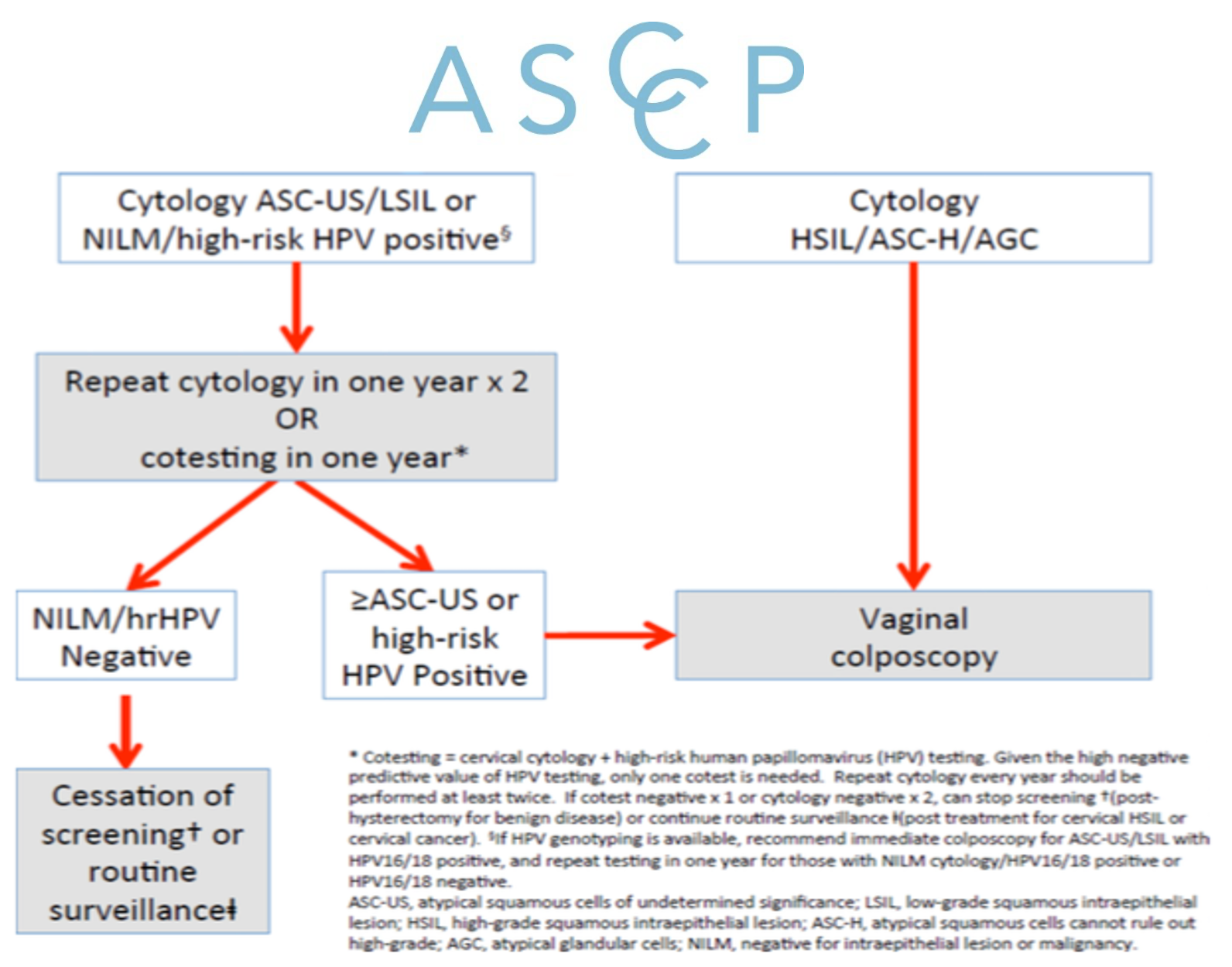
FIGURE 1
For individuals with persistent cytologic LSIL (beyond 2 years without progression to cytologic HSIL) consider extending the screening and colposcopy intervals to every 2-3 years. There are people (immunosuppressed or otherwise) who are not able to eradicate HPV and remain at risk.
If the biopsy result is HSIL/VaIN2/3, treatment based on current best practice is recommended. This could potentially include laser ablation, topical treatment, or excision with referral to gynecologic oncology for large or complex lesions. A biopsy result of invasive carcinoma warrants referral to gynecologic oncology.
Further reading:
Khan MJ, Massad LS, Kinney W et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016 May; 141(2): 364–370. doi: 10.1016/j.ygyno.2015.11.023
Kesic V, Carcopino X, Preti M, et al. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD), and the European Federation for Colposcopy (EFC) consensus statement on the management of vaginal intraepithelial neoplasia. Int J Gynecol Cancer. 2023 Apr 3;33(4):446-461.
Standard Abbreviations
HPV - Human Papillomavirus
hrHPV - High-Risk Human Papillomavirus
HSIL - High-Grade Squamous Intraepithelial Lesion
VaIN - Vaginal Intraepithelial Neoplasia
NCCN - National Comprehensive Cancer Network
LSIL - Low-Grade Squamous Intraepithelial Lesion
NILM - Negative for Intraepithelial Lesion or Malignancy
ASC-H - Atypical Squamous Cells, Cannot Rule Out High-Grade Lesion
ASC-US - Atypical Squamous Cells of Undetermined Significance
AGC - Atypical Glandular Cells
AIS - Adenocarcinoma In Situ
LEEP - Loop Electrosurgical Excision Procedure
ECC - Endocervical Curettage
CIN - Cervical Intraepithelial Neoplasia
SCJ - Squamocolumnar Junction
mRNA - Messenger Ribonucleic Acid
CO2 - Carbon Dioxide
WHO - World Health Organization
Download PDF