Tips for Best Practice on Role of Expedited Treatment
Post date: November 15, 2024
Reviewed by: Kelsey C. Schmidt, MD
Patients with the highest risk of having cervical precancer based on clinical factors such as age, prior screening history, current cervical cytology, and HPV status including HPV genotype, have the greatest need of preventive treatment. This can be facilitated with the single visit approach of expedited treatment, where a confirmatory biopsy is not required prior to treatment. Colposcopy should still be performed, however, at the time of treatment to guide treatment decisions.
The 2019 Risk-Based Management Consensus Guidelines utilize “clinical action thresholds” to guide management of abnormal cervical cancer screening results based on the calculated immediate risk of cervical intraepithelial neoplasia (CIN) 3+. The clinical action threshold of “Expedited Treatment Preferred” is met when the calculated immediate risk of CIN 3+ is 60% or greater for non-pregnant patients 25 years or older. The clinical action threshold of “Expedited Treatment or Colposcopy Acceptable” is met when the calculated immediate risk of CIN 3+ is between 25% and 59% for non-pregnant patients 25 years or older. Expedited treatment is not recommended for pregnant patients or those under age 25 years.
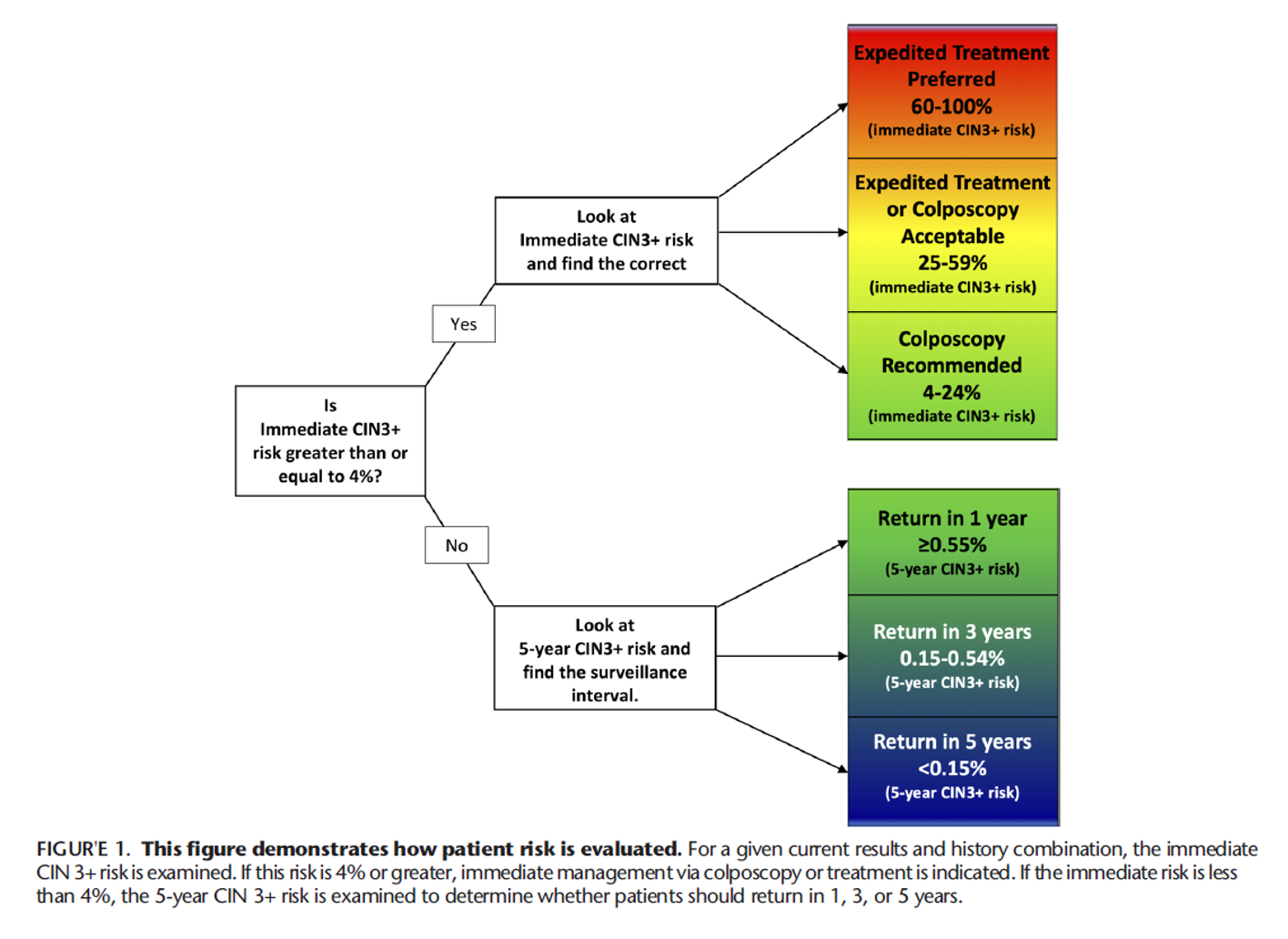
Example of clinical scenarios that reach an “Expedited Treatment Preferred” threshold include nonpregnant patients 25 years or older with high grade squamous intraepithelial lesion (HSIL) cytology and concurrent positive testing for human papillomavirus genotype 16 (HPV 16) (immediate CIN 3+ risk 60%) or never/rarely screened patients (no screening in >5 years) with HPV-positive HSIL cytology, regardless of genotype (immediate CIN 3+ risk 64%). Examples of clinical scenarios that reach an “Expedited Treatment Acceptable” threshold include HPV-positive atypical squamous cells cannot rule out high grade (ASC-H) cytology (immediate CIN 3+ risk 26%), HPV-negative HSIL cytology (immediate CIN3+ risk 25%), HPV-positive atypical glandular cells (AGC) cytology (immediate CIN 3+ risk 26%), and HPV-positive HSIL cytology (immediate CIN 3+ risk 49%).
Clinicians should employ shared decision-making with a thorough discussion regarding risks and benefits when considering expedited treatment, especially for patients with concerns about the potential consequences of treatment on future pregnancy outcomes. Expedited treatment may be preferable to patients for a variety of reasons including personal preference, limited access to healthcare, financial concerns, and cancer-related anxiety.
Further reading:
Perkins RB, Guido RS, Castle PE et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-31.
Schiffman M, Wentzensen N, Perkins RB, Guido RS. An Introduction to the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis. 2020;24(2):87-89.
Zheng Z, Yang X, Yao X, Li L. Prognostic value of HPV 16/18 genotyping and geminin mRNA quantification in low-grade cervical squamous intraepithelial lesion. Bioengineered. 2021 Dec;12(2):11482-11489. doi: 10.1080/21655979.2021.2009959. PMID: 34874226; PMCID: PMC8810151.
Standard Abbreviations
HPV - Human Papillomavirus
hrHPV - High-Risk Human Papillomavirus
HSIL - High-Grade Squamous Intraepithelial Lesion
VaIN - Vaginal Intraepithelial Neoplasia
NCCN - National Comprehensive Cancer Network
LSIL - Low-Grade Squamous Intraepithelial Lesion
NILM - Negative for Intraepithelial Lesion or Malignancy
ASC-H - Atypical Squamous Cells, Cannot Rule Out High-Grade Lesion
ASC-US - Atypical Squamous Cells of Undetermined Significance
AGC - Atypical Glandular Cells
AIS - Adenocarcinoma In Situ
LEEP - Loop Electrosurgical Excision Procedure
ECC - Endocervical Curettage
CIN - Cervical Intraepithelial Neoplasia
SCJ - Squamocolumnar Junction
mRNA - Messenger Ribonucleic Acid
CO2 - Carbon Dioxide
WHO - World Health Organization
Download PDF